Medical devices, smart drug delivery, wearables and technology for the treatment of Diabetes Mellitus
Daniel A Domingo-Lopez*, Giulia Lattanzi*, Lucien HJ Schreiber*, Eimear J Wallace*, Robert Wylie, Janice O’Sullivan, Eimear B Dolan, Garry P Duffy
* These authors contributed equally to this work.

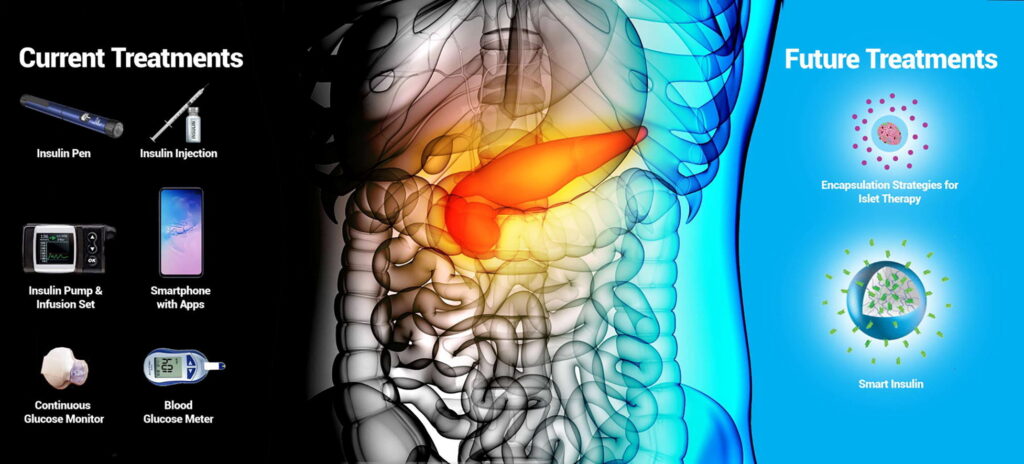
Abstract: Diabetes mellitus refers to a group of metabolic disorders which affect how the body uses glucose impacting approximately 9% of the population worldwide. This review covers the most recent technological advances envisioned to control and/or reverse Type 1 diabetes mellitus (T1DM), many of which will also prove effective in treating the other forms of diabetes mellitus. Current standard therapy for T1DM involves multiple daily glucose measurements and insulin injections. Advances in glucose monitors, hormone delivery systems, and control algorithms generate more autonomous and personalised treatments through hybrid and fully automated closed-loop systems, which significantly reduce hypo- and hyperglycaemic episodes and their subsequent complications. Bi-hormonal systems that co-deliver glucagon or amylin with insulin aim to reduce hypoglycaemic events or increase time spent in target glycaemic range, respectively. Stimuli responsive materials for the controlled delivery of insulin or glucagon are a promising alternative to glucose monitors and insulin pumps. By their self-regulated mechanism, these “smart” drugs modulate their potency, pharmacokinetics and dosing depending on patients’ glucose levels. Islet transplantation is a potential cure for T1DM as it restores endogenous insulin and glucagon production, but its use is not yet widespread due to limited islet sources and risks of chronic immunosuppression. New encapsulation strategies that promote angiogenesis and oxygen delivery while protecting islets from recipients’ immune response may overcome current limiting factors.
